Make an Electroscope
To better understand electricity, start with understanding electrostatics which is the study of electric charges at rest.
Conductors and Insulators
Materials are generally considered to be either conductors or insulators, based on their ability to conduct or block electricity.
Metals such as copper and iron for example are classified as conductors, because some of the electrons present in these materials are free to move around the entire material. These are materials which transmit electricity; however some conductors transmit electricity better than others. Copper and silver are some of the best conductors e.g. metals, carbon and water (Note that if the water is a good conductor only if it contains some impurity because pure water is not a good conductor).
On the other hand, the electrons present in a piece of glass or a chunk of wood for example are bound to nearby atoms; this means that these electrons will not be able to move freely inside the material. For this reason, materials such as glass and wood are classified as insulators.
It is possible to detect and measure the electric charge on a material by using an electroscope, shown below:
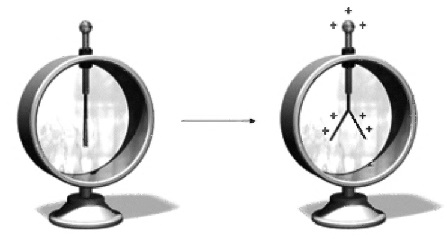
How to make your own Electroscope:
You can build your own electroscope at home using simple things you can find around the house, such as:
- a glass jar,
- 2 little pieces of aluminium foil cut into small round shapes (and you need to put a whole in one end of each foil);
- A copper wire (spiral one of the ends of the wire to make a large surface area);
- Some electrical tape,
- A plastic lid cut into a circle the same size as the jar opening (you can use the plastic lid from any plastic container);
- A small plastic tube (you can use a straw) shoved through the plastic lid;
The components of the electroscope are shown below:

To assemble the electroscope, take the copper wire and slide it through the plastic tube and make a little hook at the bottom end of the copper wire. Put the 2 pieces of foil on the hook one after the other using the hole that you made on each of the pieces of foil. The 2 pieces of foil should now be touching together at the end of the hook.
Take the plastic lid and put it on top of the jar and secure it with some electrical tape to make sure the lid is firmly fixed on the top of the jar. When you’re done, you should have something that looks like the home-made Electroscope shown below:

To test the Electroscope, use a small piece of vinyl and rub it for a few seconds with the palm of your hand, then move this piece close to the copper wire edge. Watch how the aluminium pieces spread apart!
So what is it that makes the aluminium pieces spread apart in this experiment?
The reason this works is because when you rub your hands against a piece of material such as plastic or vinyl for example, electrons from your hands will get transferred to that material which means that the piece will become more negative (or negatively charged) and your hands will become more positive. When you move the vinyl piece closer to the electroscope, this will cause the electrons that are on the piece of copper to move down the tube and away from the piece of vinyl because similar charges repel each other. When the electrons are pushed down the copper wire, they will be transferred down into the hook and into the pieces of foil. At this stage, both pieces of aluminium foil have become negatively charged and since negative charges repel negative charges, this explains why the 2 aluminium pieces spread apart!
Learn more about electronics or science in general by following the links below -
Electronics
Science
[14/12/2025 11:45:54]